With approximately 100000 Depuy hip replacement patients at risk and now subject to a recall of their Depuy ASR hip replacements a voluntary and independent database was created of hip replacement patients. Hip replacement lawsuits claim patients were left with serious side effects due to device design or defects.
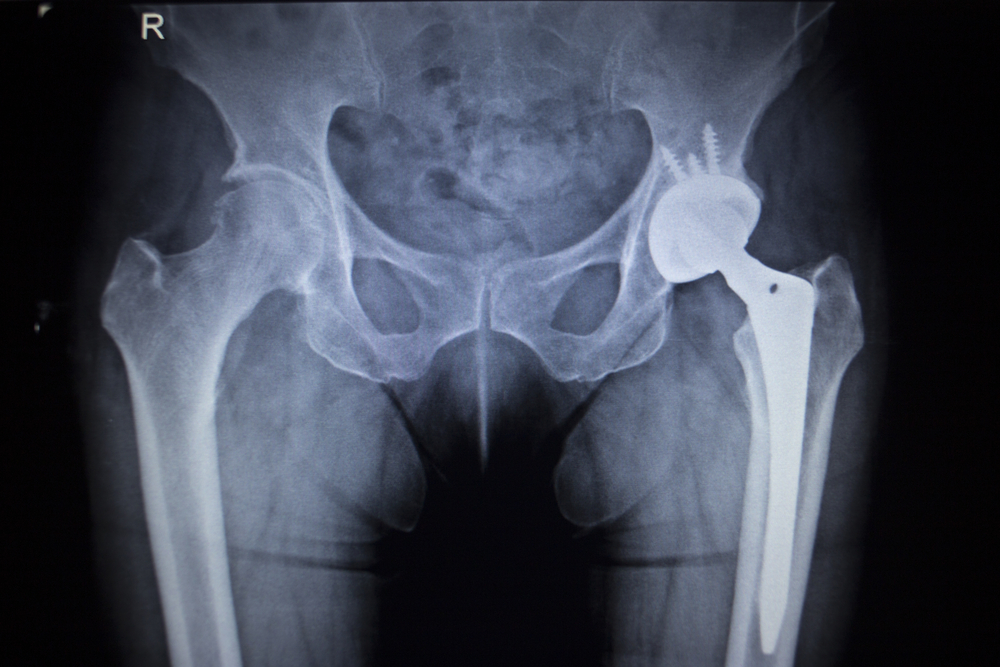
Hip Replacement Recall Recall Process Causes
A hip replacement involves removing a worn or damaged hip joint and inserting an artificial version in its place.

. Liability for hip replacement recalls rests with the manufacturer. Liability for Hip Replacement Recalls. A hip replacement recall claim can help you acquire the compensation you deserve.
This is the second time there has been a recall and this is why. March 13 2022 An X-ray of the pelvic area showing a replacement hip. Hip replacement device manufacturers have ponied up more than 7 billion to reach settlements with victims.
In which the authors studied corrosion at the modular head-neck junction as a cause for early failure in modern hip replacement systems. The authors analyzed a large revision retrieval database of femoral stems and among other things concluded that one of the strongest predictors of corrosion was the flexural. Medical Professionals mentioned that the DePuy Articular Surface Replacement ASR hip systems need to happen to be recalled right away when complaints on metal-on-metal hip implants started increasing.
Depuy Asr Hip Replacement Ought to Happen to be Recalled Earlier. An x ray of a hip joint problem - hip replacement stock pictures royalty-free photos images. Side effects include allergic reactions metal poisoning infection nerve damage and bone loss.
Hip replacement surgery replaces part of the hip joint called the ball-and-socket with artificial materials. See more ideas about hip replacement hip implants hips. Hip replacement is a surgical procedure in which the hip joint is replaced by a prosthetic implant that is a hip prosthesisHip replacement surgery can be performed as a total replacement or a hemi half replacement.
If you have received a hip replacement you need to know about these recalls and what steps to take if your hip fails or you find out. Hip replacement surgery is one of the most common and most successful orthopedic surgeries performed today. Hip-Implant It has been recently revealed in a report that there is need for an inquiry to be conducted on the recalls being done on the hip replacement devices.
These recalls came too late for tens of thousands of people who already received the problematic devices. Beginning in 2005 Johnson Johnson distributed its DePuy ASR XL Acetabular System. In most cases the manufacturer will bear sole liability for any losses you faced as a result of your hip replacement recall.
It is very expensive for the FDA to do a recall of medical devices like those used in hip replacement surgeries which is probably why they have only done three in a 10-year period 1993-2003. Contact Sokolove Law Now. Victims who filed Hip implant lawsuits often needed a 2nd surgery.
Ad Exactech Has Recalled Many Hip Replacement Products. DePuy described the ASR XL as a high performance hip replacement advertising it with pictures of a young woman running on a sandy beach and a man taking a very aggressive golf swing. Other patients have developed complications after undergoing a hip replacement with a Stryker Tritanium Acetabular Shell.
Most people who undergo surgery for hip replacement experience positive outcomes in their daily life like better mobility reduced pain a better quality of life and improvements in their daily activities. Metal plastic and ceramic can all be used to replace the hip joint. Known settlements amount to at least 22 billion.
What is the Hip Replacement Recall And How Does It Affect Oklahomans. Update-11212020- A jury in Federal Court in St. Over 600000 hip replacement surgeries are performed annually in the United States.
The shells or cups sometimes loosen after surgery causing patients to. Weve Recovered Over 84 Billion For Our Clients. Biomet DePuy Smith Nephew Stryker Wright and Zimmer.
Although hip replacement problems are not frequently experienced there are a number of possible complications following hip replacement surgery. An unusual recall of a hip replacement device has sparked class action lawsuits in Canada and the US with some patients wondering why it. Depuy ASR Implant patients are urged to Register with the National Hip Recall Registry.
Louis failed to grant punitive damages to a victim of a defective hip replacement device. Stryker had the most recalls with 231 and DePuy came in second with 150. Such joint replacement orthopaedic surgery is generally conducted to relieve arthritis pain or in some hip fracturesA total hip replacement total hip arthroplasty or.
This type of surgery is typically done when damage from arthritis injury or other conditions have resulted in enough pain and lack of mobility to make everyday life challenging. The company advertised many advantages of the ASR XL hip replacement system over both conventional hip replacements and over hip resurfacing. Hip replacement recalls have followed with some companies voluntarily recalling their hip systems and components.
Jan 27 2016 - Our team of affiliated attorneys can help you with your metal hip recall case semi truck accident and car accidents. Browse 817 hip replacement stock photos and images available or search for hip replacement surgery or hip replacement patient to find more great stock photos and pictures. Jan 22 2012 by admin.
From 2002 to 2013 Consumers Union found 578 recalls from six major manufacturers. Since the majority of people who undergo this surgery are suffering from hip pain that was caused by hip arthritis the absence or minimization of pain after. Hip replacement surgery is a major procedure but one that has helped many people get relief from pain and enjoy mobility again.
If you have had a hip replacement procedure you may be entitled to a second revision surgery and costs for pain and suffering due to a defective medical device. Stryker recalled two of its hip replacement systems that contained the alloy. Ceramic is a high.
DePuy in August 2010 announced a recall of its hip implant device after reports showed that an unusually high number of people using the companys products were in need of revision surgery andor a second hip replacement. The products in question are the DePuy ASR Hip Resurfacing System and the DePuy ASR XL Acetabular System. When companies do it themselves they can recall for any reason marketing defects patient complaints insufficient instructions for surgeons and more.
Problems caused by arthritis can be treated with hip replacement surgeryBroken hips hip fractures avascular necrosis and other conditions can. Stryker LFIT V40 Recall. Thousands of hip replacement lawsuits have been filed against various device makers.
Biomet Hip Replacement Recall Lawsuit

Stryker Hip Implant Recall Rejuvenate Abg Ii Complications
Defective Hip Implants Have Been Recalled By Several Manufacturers Attorneys Are Filing Defective Hip Implant Lawsuits
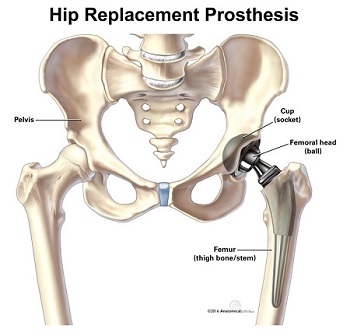
Stryker Hip Prosthesis Recall 2016 Hip Replacement Lawsuit Attorneys

Stryker Hip Implant Recall Gray And White Law

Defective Hip Implants Lawsuit Attorney Jonathan Kline P A
Stryker Hip Replacement Recall Lawyer Hip Implant Lawsuit Attorney
0 komentar
Posting Komentar